Light is something we interact with every day, whether it’s sunlight illuminating the sky or artificial light inside our homes. However, what we don’t often realize is that light, in addition to illuminating the world around us, also carries energy and momentum. These aspects of light are not only crucial for understanding its behavior but also for exploring some of the most advanced concepts in modern physics and technology. In particular, light’s momentum can take the form of linear momentum, which is responsible for how a speeding train is difficult to stop, and orbital angular momentum, which governs how objects like the Earth maintain their orbit as they revolve around the Sun.
In recent research, scientists have taken a significant step toward leveraging light’s orbital angular momentum to manipulate electrons. Their work, published in Nature Photonics on November 26, 2024, demonstrates that a beam of light can reliably transfer orbital angular momentum to electrons, specifically those in graphene. This discovery has important implications for controlling quantum interactions, a key requirement for advancing technologies like quantum computing and quantum sensing. The transfer of orbital angular momentum to electrons could offer a new method for manipulating the quantum states of matter, pushing the boundaries of how we interact with electrons at the quantum level.
The concept of orbital angular momentum in light is an intriguing one. Light typically travels as a wave, oscillating in electric and magnetic fields. The fundamental property of light carrying orbital angular momentum means that as light moves through space, its wavefronts twist in a spiral fashion around an axis. This twisting creates a vortex at the center of the light beam, a characteristic that can be harnessed to influence matter, especially in the field of quantum physics. For years, scientists have been interested in how light with orbital angular momentum can interact with matter, but experimental demonstrations of this phenomenon have been few and far between. A key challenge has been the mismatch in scale between light and the particles it is intended to influence.
Electrons, which are much smaller than the wavelength of visible light, are typically too small to directly interact with the changes in the wavefront of light that carries orbital angular momentum. The size of the light beam is typically far larger than an atom or the electrons orbiting it, making it difficult to transfer momentum or angular momentum effectively. Photons—the particles that make up light—carry energy and momentum, but the wavelengths of visible light are much too large for direct interaction with electrons inside atoms, which are approximately 1,000 times smaller than the wavelengths of light. As a result, while light can transfer energy to atoms, transferring angular momentum to individual electrons, especially the orbital angular momentum, presents significant challenges.
One possible solution to this problem is to shrink the wavelength of the light. However, this would increase the energy of the photons, making it difficult for atoms to interact with them effectively. Instead of reducing the wavelength, researchers in this new experiment adopted an alternative approach: they expanded the size of the electrons themselves. Instead of working with electrons bound tightly to atomic nuclei, the researchers turned to graphene, a material that allows electrons to move freely over much larger distances while still remaining under some form of control. This made the electrons more responsive to the subtle effects of light with orbital angular momentum.
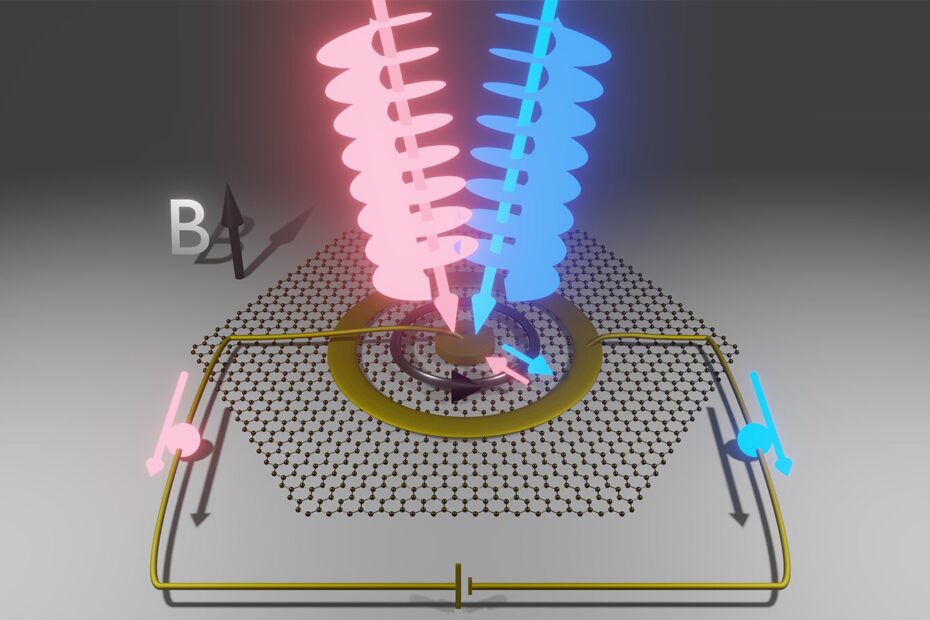
Graphene, a two-dimensional sheet of carbon atoms arranged in a honeycomb structure, is one of the best electrical conductors known to science. This remarkable material was chosen because it allows electrons to move easily across its surface, but it also presents the opportunity to manipulate these electrons in ways that can’t be done with traditional materials. The researchers took a sample of graphene and cooled it to just 4 degrees above absolute zero. They then applied a strong magnetic field, which caused the electrons to enter what are called cyclotron orbits, where they move in tight, circular paths around the magnetic field lines. This effect allows electrons to move in larger loops than they could inside individual atoms, making them more sensitive to the influence of light carrying orbital angular momentum.
With this setup in place, the team performed a series of experiments to test whether they could induce a current by transferring angular momentum from light to the electrons. The graphene sample was equipped with electrodes, with one in the center and another forming a ring around the edges of the sample. The theoretical framework, developed in 2021 by a former graduate student and co-author of the study, Bin Cao, suggested that electrons in cyclotron orbits could gain orbital angular momentum in chunks when exposed to light carrying this form of momentum. These angular momentum exchanges would alter the size of the electron orbits and potentially drive a current across the material.
Indeed, the results were promising. When exposed to light with clockwise orbital angular momentum, the current in the graphene sample flowed in one direction. When the light was switched to carry counterclockwise orbital angular momentum, the direction of the current reversed. This was exactly the behavior the researchers had expected based on the theory, confirming that the electrons were responding to the angular momentum of the light beam. In addition to this, when they altered the direction of the magnetic field, they observed a similar reversal in the direction of the current, demonstrating that the electrons’ motion could be manipulated by the light’s orbital angular momentum.
The researchers also tested whether circularly polarized light, which carries intrinsic angular momentum but not orbital angular momentum, would have a similar effect. Interestingly, they found that circularly polarized light did not induce a significant current, suggesting that it is the orbital angular momentum carried by the light that is critical to driving the current in this experiment. This result provides further confirmation that the behavior was due to the light’s orbital angular momentum rather than its other properties.
The success of this experiment marked the culmination of years of work, overcoming challenges in both the fabrication of the graphene samples and the alignment of the light beam. Creating the right sample geometry for the experiment took over a year, with the team eventually collaborating with experts at the Polytechnic University of Milan to prepare the graphene samples. Additionally, aligning the twisted light beams with the sample proved to be a delicate task, and it was only after mapping the sample with high precision that the team was able to consistently observe the effect they were looking for.
Beyond demonstrating a new method for controlling matter with light, the results of this experiment open the door to new techniques for probing the quantum nature of electrons in materials. By combining specially prepared light beams with interference measurements, the researchers believe they could create a kind of microscope capable of imaging the spatial extent of electrons—an important step toward understanding the quantum properties of materials and manipulating them in controlled ways.
Being able to not only detect but also control the spatial properties of free electrons is seen as a crucial milestone in the field of quantum physics. Manipulating these electrons with high precision is a central goal of quantum computing and sensing, where the ability to control quantum states can lead to breakthroughs in computing power and sensitivity. The ability to manipulate orbital angular momentum could thus play an important role in advancing quantum technologies, providing new ways to control and measure the behavior of electrons in quantum materials.
Source: Joint Quantum Institute