Recent research led by investigators from Weill Cornell Medicine has uncovered a fascinating discovery that could reshape our understanding of fungal evolution within the gastrointestinal tract. The study, published in Nature, highlights the presence of a specific fungus in the stomach of wild mice, known as Kazachstania pintolopesii (K. pintolopesii). This yeast species, which thrives in the stomachs of wild mice, is not only remarkably well-adapted to its host but also has significant implications for understanding both mouse physiology and the broader role of gut fungi in human health.
The discovery sheds light on the potential role of fungal commensals—microorganisms that live symbiotically in the gastrointestinal tract—an area of study that has long been overshadowed by research on bacterial commensals. While bacteria have been extensively studied for their influence on human immunity and disease—such as their involvement in cancers, inflammatory disorders, and even mental health conditions—fungi have received far less attention. This imbalance is partially due to the lack of an appropriate mouse model that accurately mimics fungal commensalism in the gastrointestinal tract.
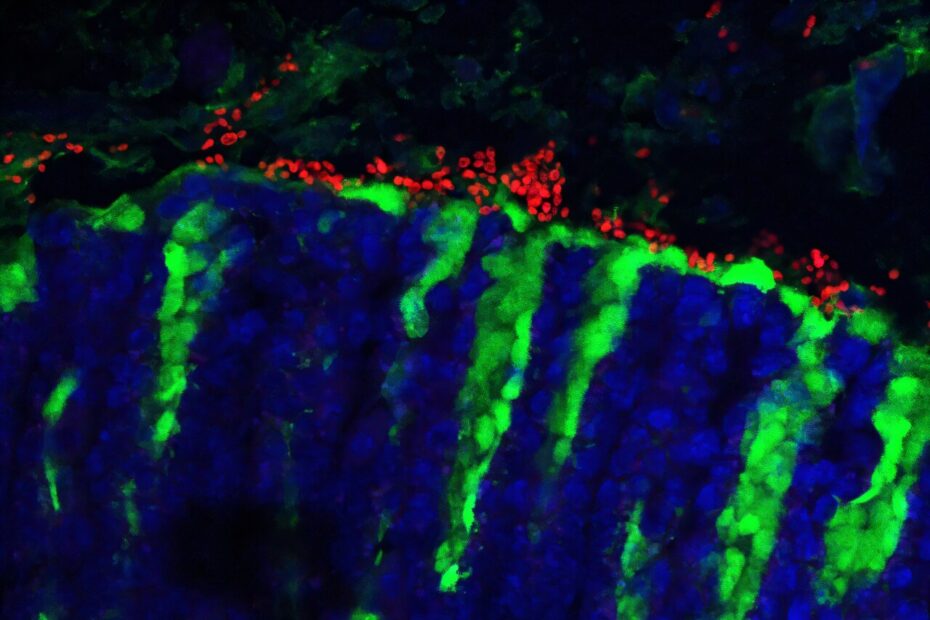
In the study led by Dr. Iliyan Iliev, a senior author and associate professor of immunology in medicine at Weill Cornell Medicine, the research team made a critical breakthrough by identifying K. pintolopesii as a dominant fungal species in the gastrointestinal tract of wild mice. The findings suggest that this fungus is not just an incidental presence but an integral part of the microbiota, providing key benefits to the host while also influencing immune responses. These revelations open new avenues for understanding the complex relationship between fungi and the immune system, which could have significant implications for how we study immune responses in preclinical research.
The researchers’ journey to discovering K. pintolopesii was anything but straightforward. Initially, fungal populations in laboratory mice were found to be transient and highly variable from colony to colony, leading scientists to question whether a true fungal commensal existed. Previous efforts had failed to identify a stable fungal population, and the species present were often transient or inconsistent. However, in 2019, a study co-led by Dr. Barbara Rehermann of the National Institutes of Health revealed that lab mice raised with gut microbes from wild mice displayed immune responses much closer to those seen in humans, sparking a new line of investigation into the fungal populations of these wild mice.
Dr. Iliev’s team, building on this work, sought to identify the dominant fungus in wild mice. They turned to samples collected from pest-control companies in major cities like New York and Los Angeles, as well as from research institutions that use lab mice. Through extensive sampling, the team determined that K. pintolopesii was not only prevalent in wild mice but also frequently found in laboratory mice colonies—often without the researchers being aware of its presence. This discovery raised an important point for researchers working with mouse models: the presence of this fungus may have significant effects on experimental outcomes.
One of the most striking findings was that K. pintolopesii could rapidly colonize the gastrointestinal tract of laboratory mice and was reliably transmitted to their offspring. Even more intriguing was the fungus’s ability to evade the host’s antifungal immune response, allowing it to thrive in the gut. This adaptability suggests that K. pintolopesii has evolved specifically to live in mice, making it a true commensal organism, unlike many other fungi that may only transiently colonize the gut.
The research team also discovered that this fungus could influence immune responses in mice. Under normal circumstances, K. pintolopesii suppresses the growth of other fungal species in the gastrointestinal tract. However, when the gastrointestinal environment fluctuates—due to dietary changes or antibiotic treatments—the fungus activates the production of a cytokine called IL-33. This cytokine, in turn, triggers a type 2 immune response, which is typically associated with defending against parasites and helminths (worms). While this response provides protection against parasites, it also has the downside of exacerbating allergic reactions, including food allergies. These dual effects make K. pintolopesii a unique and fascinating model organism for studying the role of fungi in immune responses.
For researchers studying allergies, parasitic infections, or immune responses linked to type 2 and type 17 immunity, K. pintolopesii could have a significant impact on their findings. As Dr. Iliev notes, “If you’re using mice to research allergies, parasite infections, cancer development, or any other area where type 2 or type 17 immune responses are relevant, then this fungus may be an important factor that you shouldn’t omit.”
Beyond its implications for mouse models, the discovery of K. pintolopesii raises broader questions about the role of fungal commensals in humans. Could a similar fungus exist in the human gut, playing a comparable role in promoting immune responses? Is there a fungal counterpart that helps regulate immunity in humans, as K. pintolopesii does in mice? These questions are currently under investigation, with Dr. Iliev’s lab actively exploring fungal populations in both humans and animals.
The study also underscores the importance of understanding the microbiota as a whole, rather than focusing on individual microbial species. The gut microbiota is a complex ecosystem of bacteria, fungi, and other microorganisms, all of which interact in ways that influence host health. The findings of this study emphasize that researchers should consider the full range of microbial species—bacteria, fungi, and others—when conducting experiments with mice, particularly when studying immune responses. Fungi like K. pintolopesii could have a significant impact on experimental outcomes, and their presence or absence could alter the conclusions of many studies.
The research team’s efforts are far from over. Dr. Iliev and his collaborators, including teams from the Broad Institute, the National Institutes of Health, and Pennsylvania State University, are continuing to investigate fungal populations in both mice and humans. Their goal is to better understand the role of fungi in immune regulation and to explore whether K. pintolopesii or similar fungi could be harnessed for therapeutic purposes. In particular, they are interested in whether manipulating fungal populations in the gut could help treat diseases related to immune dysfunction, such as allergies, autoimmune diseases, or even cancer.
Source: Weill Cornell Medical College