A research team at the Institute of Materials Chemistry at TU Wien, led by Professor Dominik Eder, has made a significant breakthrough in the development of durable, conductive, and catalytically active hybrid framework materials for (photo)electrocatalytic water splitting. This groundbreaking study, published in Nature Communications, offers a new synthetic approach that could greatly enhance the efficiency of hydrogen production through water splitting—a process central to sustainable energy generation.
Hydrogen (H2) is widely regarded as a promising clean energy carrier, with water splitting—either electrochemically or with the aid of light—being one of the most efficient methods of producing it. The challenge, however, lies in identifying a suitable catalyst that can accelerate the reaction without being consumed in the process. An effective catalyst for water splitting must possess two key characteristics: a large surface area to facilitate the adsorption and splitting of water molecules, and exceptional durability for long-term performance.
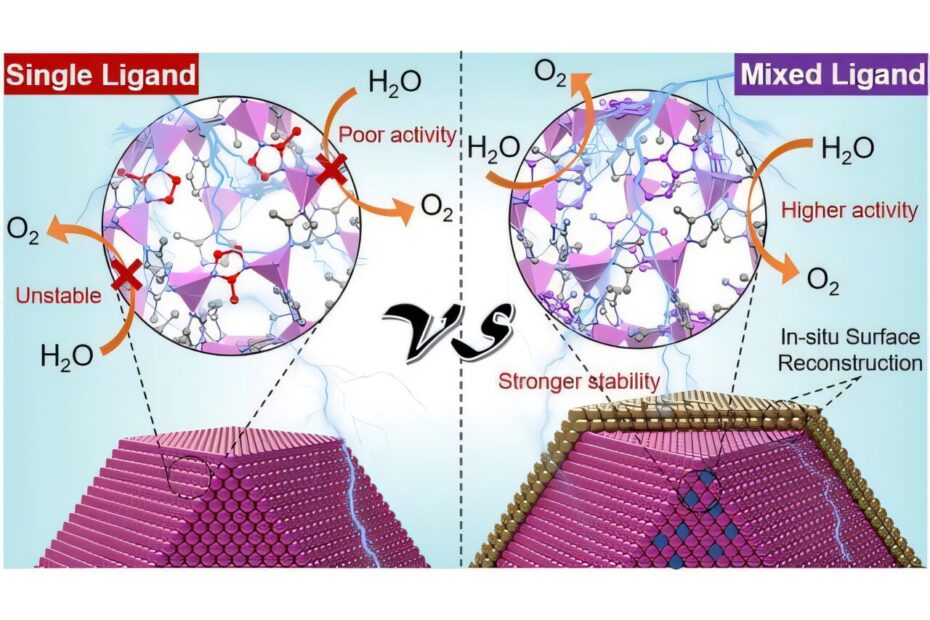
Zeolitic imidazolate frameworks (ZIFs), a class of hybrid organic/inorganic materials, offer a potential solution to these challenges. ZIFs are composed of metal ions, such as cobalt, coordinated with organic ligands through coordination bonds. These frameworks possess an extremely high surface area and numerous porous sites that can adsorb water molecules, making them excellent candidates for catalytic applications. However, conventional ZIFs suffer from two significant drawbacks: they often lack stability in aqueous environments under electrocatalytic conditions, limiting their long-term use, and their low electronic conductivity restricts their efficiency in electrocatalytic processes.
To overcome these issues, Professor Eder and his team developed an innovative strategy to enhance ZIF stability and conductivity. By modifying the ZIFs to incorporate two or more different organic ligands, they aimed to optimize the material’s properties. This approach required careful selection and mixing of ligands to ensure that they were evenly distributed throughout the framework, while still preserving the integrity of the original ZIF structure. Through extensive investigations into various ligand combinations and processing parameters, the team identified the optimal ligand pair for the new hybrid framework.
The results were striking. The team discovered that the dual-ligand approach significantly improved the stability of the ZIFs, extending their durability during electrocatalytic water splitting from just a few minutes to at least one day. Using a range of experimental spectroscopic and microscopic techniques, combined with computational simulations in collaboration with Central China Normal University, the researchers found that the interaction between the two ligands strengthened the coordination bond with the cobalt metal, which in turn prevented the framework from collapsing during catalysis.
Interestingly, the dual-ligand modification also led to the formation of a thin protective cobalt oxyhydroxide film on the surface of the ZIF nanoparticles after just a few minutes of the reaction. This film acted as a shield, preventing further degradation and collapse of the material. Moreover, the conductivity of the new ZIF material increased by a factor of 10, which in turn boosted the oxygen evolution reaction (OER) rate by a remarkable factor of 10 as well. This improvement in both conductivity and catalytic efficiency was attributed to the synergistic interaction between the two ligands, which created a high density of mobile charge carriers throughout the material.
According to Professor Eder, the simulations revealed that the dual-ligand approach was more effective than initially expected, leading to a significant enhancement in the (photo)electrocatalytic performance of the ZIFs. “Although we expected some improvements with this new strategy, we were surprised by how much it enhanced the (photo)electrocatalytic performance of ZIFs,” he commented. The combination of improved stability, increased conductivity, and enhanced catalytic activity could pave the way for more efficient hydrogen production technologies.
Building on the success of this new approach, the team is now exploring the potential for applying this technique to other types of ZIFs and metal-organic frameworks (MOFs), which also face issues with stability and conductivity in electrocatalytic applications. By improving these materials, the research team aims to develop advanced catalysts not only for water splitting, but also for a wide range of applications in catalysis, sensing, and solar energy conversion.
This work represents a significant step forward in the development of materials for sustainable energy technologies. By addressing key challenges such as stability, conductivity, and catalytic efficiency, the team’s innovative approach moves us closer to realizing practical, large-scale hydrogen production systems. The research also opens new possibilities for the design of advanced materials that could have broad applications in renewable energy, contributing to the transition toward a more sustainable future.
Source: Vienna University of Technology