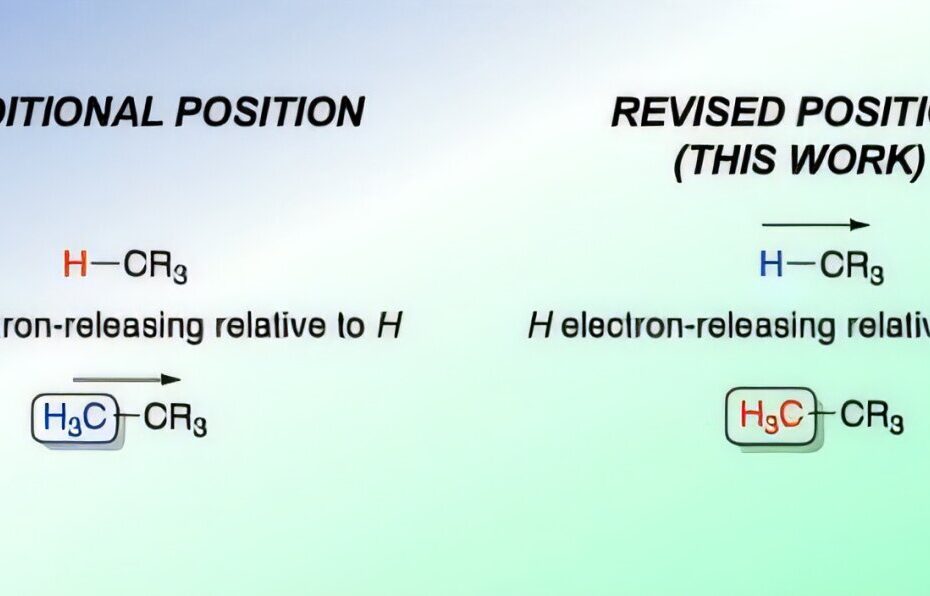
For nearly a century, chemistry textbooks have incorrectly described the behavior of alkyl groups in organic molecules, leading to widespread misunderstanding in the field. Alkyl groups, which are chains of carbon and hydrogen atoms, have long been believed to donate electrons to other parts of a molecule. However, a groundbreaking study led by Dr. Mark Elliott from Cardiff University’s School of Chemistry has overturned this longstanding belief, revealing that alkyl groups actually pull electrons away from the rest of the molecule, making them electron-withdrawing instead of electron-donating, as previously thought.
This revelation is based on the results of advanced computational methods that analyzed the distribution of electrons in molecules containing alkyl groups. These findings, published in the journal Organic and Biomolecular Chemistry, challenge a fundamental concept that has been central to structural organic chemistry for almost 100 years. The paper, titled “Alkyl groups in organic molecules are NOT inductively electron-releasing,” presents a new understanding of alkyl group behavior that could reshape how chemists approach the reactivity of organic compounds.
The concept of electron donation or withdrawal is essential to understanding chemical reactivity. Traditionally, alkyl groups were thought to donate electrons through an inductive effect, which would influence the behavior of other parts of the molecule. This inductive donation was key to explaining various molecular properties and reactivity patterns. However, the Cardiff team’s research demonstrates that alkyl groups actually withdraw electrons, a finding that has significant implications for the study and teaching of organic chemistry.
Dr. Elliott explained that this misconception had been perpetuated for decades in textbooks, which often relied on the incorrect assumption that alkyl groups were electron-donating. The discovery that alkyl groups are electron-withdrawing compared to hydrogen atoms, as shown by their study, is a crucial update that will require textbooks and educational materials to be revised. This shift in understanding is significant, as it affects the interpretation of a wide range of molecular behaviors, including reaction mechanisms, stability, and the design of new chemical compounds.
Reflecting on his own surprise at discovering the inconsistencies in the traditional view, Dr. Elliott shared that he had begun to question the long-held belief after encountering data that didn’t align with the standard explanation. His doubts were validated by the literature, which also contained hints that others had previously questioned the inductive effects of alkyl groups, but their findings had not gained the traction needed to change the widely accepted theory.
The implications of this discovery go beyond the correction of a single concept. Understanding the true inductive effect of alkyl groups is crucial for properly contextualizing all other effects within organic molecules. With this new information, chemists will be better equipped to predict and explain the behavior of organic compounds, especially in the context of chemical reactions, molecular design, and materials science.
As this research challenges an established cornerstone of organic chemistry, it highlights the evolving nature of scientific understanding. The study’s findings emphasize the importance of questioning long-standing theories and pursuing rigorous, data-driven research to refine and advance our understanding of the molecular world.
This correction in the field of organic chemistry may seem small, but it has profound implications for both research and education. The fact that nearly every organic chemistry textbook will need to be updated to reflect these new findings marks a pivotal moment in the field, as textbooks and educational practices adapt to incorporate a more accurate understanding of the role of alkyl groups in chemical reactions.
Source: Cardiff University