Researchers from Helmholtz Munich and Ludwig-Maximilians-Universität (LMU) have uncovered a potentially key mechanism underlying the neurological symptoms observed in long COVID patients. The study reveals that the SARS-CoV-2 spike protein, which facilitates the virus’s entry into human cells, remains present in the brain’s protective layers and skull bone marrow for up to four years after infection. This persistent presence may be a contributing factor to chronic inflammation and could significantly increase the risk of neurodegenerative diseases.
The research team, led by Prof. Ali Ertürk at Helmholtz Munich’s Institute for Intelligent Biotechnologies, used innovative imaging techniques to identify how the spike protein lingers in specific brain regions. These findings may pave the way for targeted therapeutic strategies to mitigate the long-term neurological effects of COVID-19.
Persistent Spike Protein Accumulation in the Brain
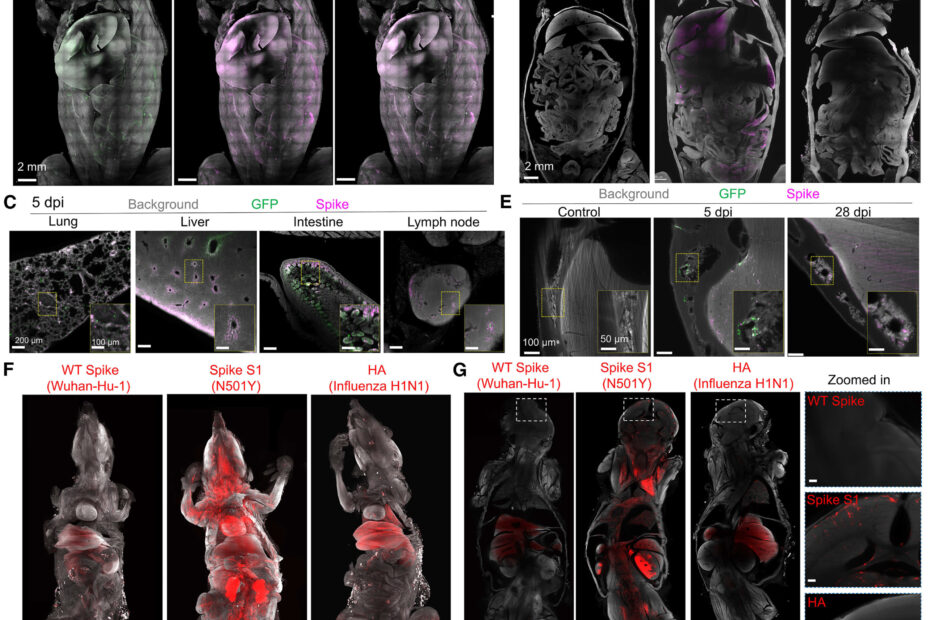
Using a novel AI-powered imaging technology, Prof. Ertürk’s team was able to render tissue samples from COVID-19 patients and mice transparent, enabling the visualization of cellular structures and viral proteins in three dimensions. This groundbreaking technique revealed that the SARS-CoV-2 spike protein accumulates in the meninges (the brain’s protective layers) and the bone marrow of the skull, even long after the initial infection. This discovery was made possible by identifying previously undetectable concentrations of the spike protein in these regions, which are rich in ACE2 receptors—molecules that the spike protein binds to in order to enter human cells.
Dr. Zhouyi Rong, the study’s first author, emphasized that these brain-adjacent areas may be especially vulnerable to the long-term accumulation of the spike protein. Prof. Ertürk added that the presence of the spike protein at the brain’s borders might contribute to the neurological symptoms of long COVID, including cognitive impairment and accelerated brain aging. Their data suggests that persistent spike protein in these regions could lead to a loss of five to ten years of healthy brain function in affected individuals.
mRNA Vaccines and the Reduction of Spike Protein Accumulation
One of the most significant findings of this study is that mRNA COVID-19 vaccines, particularly the BioNTech/Pfizer vaccine, significantly reduce the accumulation of the spike protein in the brain. Mice vaccinated with the mRNA vaccine showed lower levels of spike protein in both brain tissue and skull bone marrow compared to unvaccinated mice. However, the reduction was not complete—around 50% of spike protein levels remained, indicating that while the vaccine provides crucial protection, it does not fully eliminate the risk of long-term neurological consequences.
“These results underscore the importance of vaccines in mitigating the long-term effects of COVID-19,” says Prof. Ertürk. “However, the residual spike protein still poses a potential threat, suggesting that additional treatments or therapies will be needed to address the full spectrum of long COVID-related brain inflammation.”
While the findings were derived from mouse models, the researchers stress that further studies are needed to evaluate whether these results hold true in humans. These studies could provide insight into how mRNA vaccines help reduce long COVID’s impact, which could be vital for global public health.
The Societal and Medical Challenge of Long COVID
Long COVID remains a significant health challenge worldwide, with an estimated 50 to 60 percent of the global population having been infected with COVID-19. Of these, around 5 to 10 percent develop long COVID, resulting in neurological and cognitive impairments that may last for months or even years. This translates to approximately 400 million people potentially experiencing the long-term consequences of COVID-19, with many carrying elevated levels of spike protein in their bodies.
Prof. Ertürk emphasized that long COVID is not just a personal health issue but a societal one, with wide-reaching implications for healthcare systems. Although mRNA vaccines have proven effective in reducing the risk of long-term neurological damage, the continued occurrence of infections—especially breakthrough cases—means that some individuals may still suffer from persistent spike protein accumulation. This could lead to chronic brain inflammation, an increased risk of strokes, and other neurological complications.
Advances in Diagnosis and Treatment
The study also opens new possibilities for diagnosing and treating the long-term neurological effects of COVID-19. The researchers highlight that areas like the meninges and skull bone marrow, which are prone to spike protein accumulation, are more accessible for medical examination than brain tissue itself. Protein panels and tests designed to detect specific proteins in cerebrospinal fluid or blood plasma could provide an early diagnostic tool for identifying neurological complications related to COVID-19.
“Identifying markers of inflammation and spike protein accumulation in blood or cerebrospinal fluid could enable early detection of neurological issues,” explains Prof. Ertürk. “This could lead to the development of targeted therapies and biomarkers that could treat or even prevent the neurological impairments caused by COVID-19.”
Additionally, the study’s findings underscore the need for continued research into therapeutic strategies aimed at reducing or eliminating residual spike protein in the brain. Prof. Ulrike Protzer, a leading virologist at Helmholtz Munich and the Technical University of Munich, noted the timely relevance of this study, given the ongoing global impact of COVID-19 and the increasing focus on its long-term effects. “This research sheds light on the unexpected pathways of brain invasion and long-term involvement of the host, which has profound implications for both science and society,” she said.
The study was published in the journal Cell Host & Microbe.