The Wadsworth–Emmons (HWE) reaction is a fundamental reaction in organic chemistry, widely used to create conjugated carbonyl compounds. Conjugated carbonyl compounds are used in many industries for synthesizing perfumes, plastics, and pharmaceuticals and are also involved in biological processes. Consequently, methods for improving HWE reactions are an active area of research.
One potential application of HWE reactions is to develop (E)-isomers of conjugated carbonyl compounds that are useful for synthesizing chemicals called hynapene analogs with promising anti-cancer properties. Unfortunately, traditional HWE reaction methods are sometimes inconsistent in their (E)- and (Z)-selectivity and require several steps to get further elongated compounds.
Several studies have investigated new reagents to improve the selectivity of HWE reactions. However, the reason for their enhanced selectivity has not yet been examined enough, nor has the range of substrates suitable for these Weinreb amide-type HWE reagents been fully explored. Additionally, the effect of different reaction conditions on the HWE reaction using the same substrate hasn’t been studied.
In a breakthrough, a research team from the Department of Applied Chemistry at Tokyo University of Science (TUS), Japan, led by Assistant Professor Takatsugu Murata, including Mr. Hisazumi Tsutsui and Professor Isamu Shiina from TUS, conducted a detailed study on HWE reactions and developed a robust and highly (E)-selective Weinreb amide-type HWE reaction with a broad substrate scope.
“The reaction we developed is faster than traditional methods such as the Wittig reaction and the corresponding ester-type HWE reaction, and the applicable compounds can be used in an extremely wide range of applications, including the synthesis of pharmacologically active analogs,” says Murata.
“A key achievement is the isolation of the active species in the reaction, which allows us to efficiently synthesize the important precursor for producing pharmacologically active compounds on a larger scale by preparing the active species in advance.”
Their study is published in The Journal of Organic Chemistry.

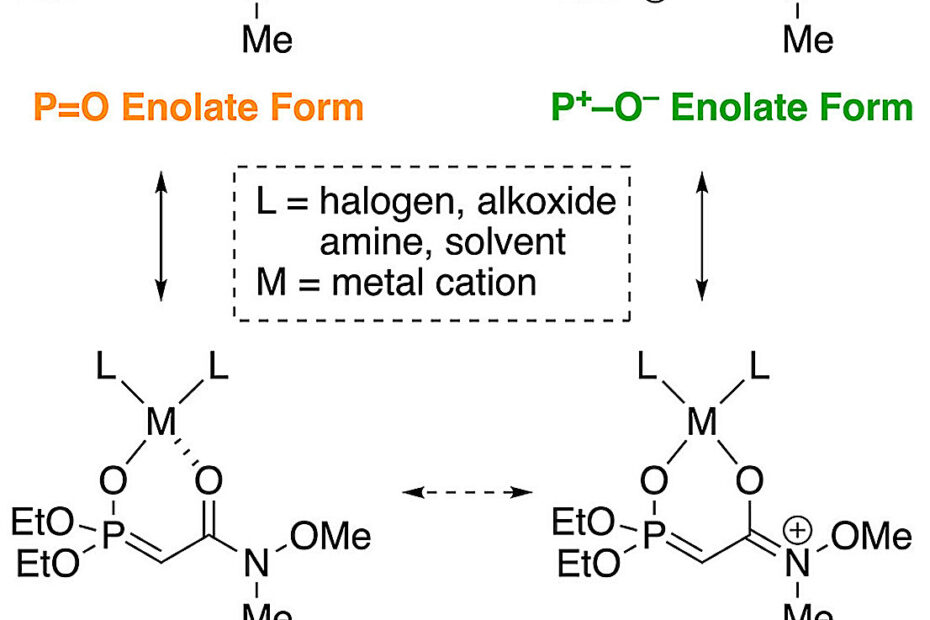
In this study, the researchers systematically tested the effect of different bases, solvents, cations, reaction concentrations, and temperatures on the reactivity and selectivity of the Weinreb amide–type HWE reaction.
They discovered that using isopropyl magnesium bromide (iPrMgBr) as a base resulted in high (E)-selectivity, thanks to the formation of a magnesium phosphonoenolate intermediate. The structure of the intermediate and the valence of the metal cation were key to improving selectivity. Moreover, replacing bromine with chlorine in the base further improved selectivity.
Interestingly, the researchers also found that the magnesium phosphonoenolate intermediate formed using the iPrMgCl base was stable enough to be isolated. This isolated intermediate was exceptionally stable, showing no deterioration when stored at room temperature in an argon atmosphere for over six months. This intermediate could be directly used in HWE reaction with high (E)-selectivity.
The team further optimized the amount of iPrMgCl, solvents, and the Weinreb amide–type HWE reagent to maximize the yield of the reaction. The optimized conditions worked well across a wide range of substrates, including various aliphatic saturated aldehydes, aliphatic a, β-unsaturated aldehydes, and aromatic aldehydes, demonstrating the robustness and scalability of the method.
To demonstrate its application, the team applied their novel reaction methodology to synthesize various complex organic compounds, including products from successive elongation processes, the HWE reaction of a cyclic ketone, and Weinreb ketone synthesis.
“Currently, hynapene analogs are being tested in various drug efficacy studies, including animal studies, and their development is highly anticipated, leading to more efficient drug development,” remarks Murata. “We are committed to improving this method further and conducting more studies to gain better insights into the reaction mechanisms.”
The researchers hope that this study offers a pathway towards novel anti-cancer drugs with potential benefits for countless patients.
Source: Tokyo University of Science