Researchers from Oregon State University (OSU) have made significant strides in carbon dioxide (CO2) capture technologies, particularly in direct air capture (DAC), which is seen as a critical strategy in combating climate change. The team, led by May Nyman and Karlie Bach from the OSU College of Science, has synthesized a new class of molecules that can efficiently capture large amounts of CO2 from the air. These findings, published in Chemistry of Materials, build on the team’s previous research with vanadium peroxides and contribute to ongoing efforts to develop more effective and cost-efficient methods for CO2 removal.
The study centers around titanium peroxides, a new material that holds promise for improving DAC technology. While current systems for capturing CO2 at the point of emission—such as those at power plants—are more developed, capturing CO2 directly from the atmosphere, where concentrations are much lower, presents significant challenges. The atmosphere’s CO2 concentration is just around four parts per million, which makes capturing it directly much more difficult compared to industrial exhaust, where the concentration is much higher.
Direct air capture technologies are still in their early stages, with only 18 active plants operating globally in the United States, Canada, and Europe. However, plans are in place to expand the number of DAC facilities to over 130 worldwide. Despite their potential, DAC technologies are costly and energy-intensive, presenting obstacles that researchers are keen to overcome. To this end, the OSU team has turned to titanium, a material that is not only more affordable and abundant than the previously explored vanadium but also safer and more environmentally friendly.
Titanium peroxides have long been studied in the context of catalysis and environmental chemistry. The OSU team sought to harness the properties of titanium in capturing CO2 in a similar way to vanadium, with the expectation that the two elements—both transition metals located near each other on the periodic table—would exhibit similar chemical behaviors. Transition metals like titanium are known for their ability to facilitate electron transfer processes, a key feature in reactions with gases such as CO2. These metals are also noted for the distinct colors they produce due to the transition of electrons between low- and high-energy states.
Bach, a graduate student in Nyman’s lab, emphasized the advantages of using titanium over vanadium. “Titanium is 100 times cheaper than vanadium, more abundant, and already has a well-established presence in industrial applications,” she said. “We hypothesized that the carbon capture behavior could be similar enough to vanadium to be effective.”
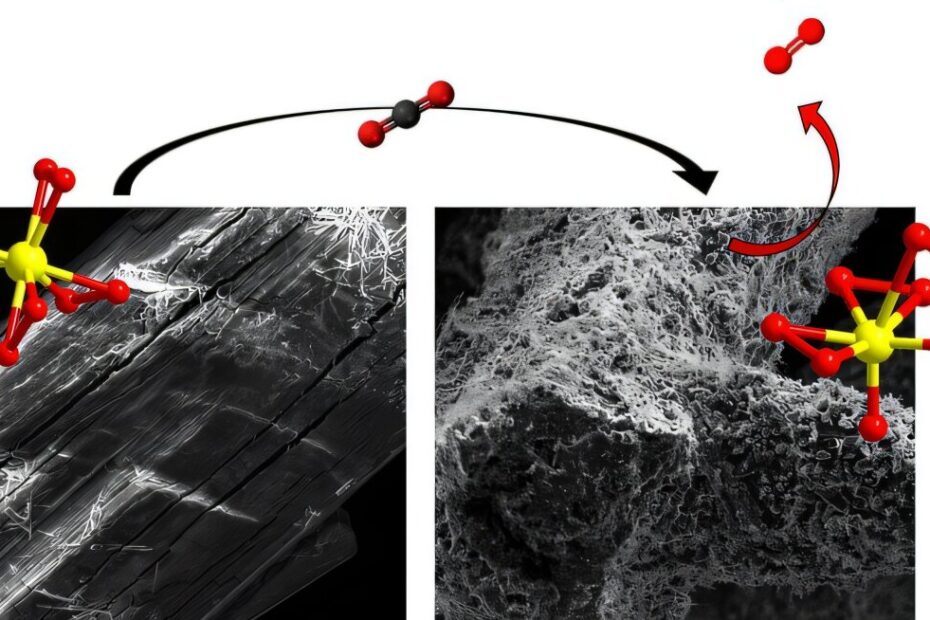
In their research, the team synthesized new tetraperoxo titanate structures, which consist of a titanium atom coordinated with four peroxide groups. These structures are highly reactive due to the peroxide groups, which are potent oxidizing agents. This reactivity is what makes the titanium-based compounds promising candidates for carbon capture. The research team was able to synthesize these compounds using low-cost materials and high-yield chemical reactions, an important step in scaling up the technology.
Among the newly synthesized compounds, the researchers found that potassium tetraperoxo titanate was particularly effective at capturing CO2. Not only was it reactive because of its peroxide groups, but it also contained hydrogen peroxide in its structure, making it even more reactive. The material’s ability to capture CO2 was measured at about 8.5 millimoles of CO2 per gram of potassium tetraperoxo titanate—roughly twice the capacity of vanadium peroxide. This high capacity, combined with the lower cost and environmental benefits of titanium, makes this new material an exciting development in the field of carbon capture.
“Titanium is a cheaper, safer material with a significantly higher capacity,” Bach noted, pointing to the compound’s potential for large-scale application. Titanium itself is an exceptionally strong yet lightweight metal that is non-toxic and resistant to corrosion. It is also the ninth most abundant element in the Earth’s crust, found in rocks, soil, plants, and even trace amounts in the human body. Its abundance and favorable properties make it a highly attractive option for use in carbon capture technologies.
The findings from OSU’s research are an important step in the development of direct air capture systems, which will likely be a necessary component of global efforts to address climate change. While technologies that capture carbon at the point of emission are already in place, DAC systems will play a crucial role in mitigating the excess CO2 already present in the atmosphere. The ability to efficiently capture and store carbon from the air is a critical tool in reducing the overall concentration of greenhouse gases, which are driving global warming.
The work of Nyman, Bach, and their team is part of a broader federal initiative to innovate new methods for DAC and develop materials capable of capturing CO2 from the air at an industrial scale. With increasing investments in these technologies and a growing urgency to address climate change, the research offers a hopeful glimpse into future solutions. By improving the cost-effectiveness and efficiency of DAC technologies, the OSU team has made a notable contribution to the global effort to combat climate change and reduce atmospheric CO2 concentrations.
Beyond the technical aspects of the research, the OSU study highlights the intersection of basic scientific research and practical applications. The team’s approach to exploring titanium as a viable alternative to vanadium demonstrates how the synthesis of new materials can directly impact the development of climate change mitigation technologies. As the world faces growing environmental challenges, the role of innovative research in creating sustainable solutions becomes increasingly important.
In addition to Nyman and Bach, the paper includes contributions from OSU assistant professors Tim Zuehlsdorff and Konstantinos Goulas, postdoctoral researcher Eduard Garrido Ribó, and several graduate students. The study also involved the expertise of Lev Zakharov, a crystallographer at OSU’s X-Ray Diffraction Facility, who assisted in characterizing the new compounds. Their collaborative efforts underscore the importance of multidisciplinary research in addressing complex global issues like climate change.
As the research continues to develop, the hope is that these new materials can be incorporated into practical DAC systems capable of removing CO2 from the atmosphere at scale. While much work remains to be done to bring these technologies to fruition, the discovery of titanium-based compounds with high CO2 capture capacities marks an important milestone in the ongoing fight against climate change. With further advancements and innovations, the dream of achieving large-scale carbon capture could soon become a reality, helping to mitigate the effects of climate change and secure a more sustainable future for generations to come.
Source: Oregon State University