Biochemists from the National University of Singapore (NUS) have made a groundbreaking discovery in the field of antimicrobial research, uncovering a new subclass of trifunctional enzymes in gram-positive bacteria. These enzymes are crucial for the biosynthesis of lanthipeptides, a class of antimicrobial peptides that have shown great promise for combating infections. Lanthipeptides, synthesized in response to environmental stress, possess a wide range of bioactivities, including antibacterial, antifungal, and antiviral properties, making them valuable candidates for the development of new drugs.
Despite their therapeutic potential, the mechanisms behind the post-translational modifications that transform these peptides into their active forms had remained largely unexplored. These modifications are critical for the peptides’ functional activity, and understanding how they occur is essential for harnessing their full therapeutic potential. The research team, led by Assistant Professor Luo Min from NUS’s Department of Biological Sciences, aimed to fill this gap in knowledge by investigating the enzymes responsible for modifying these peptides.
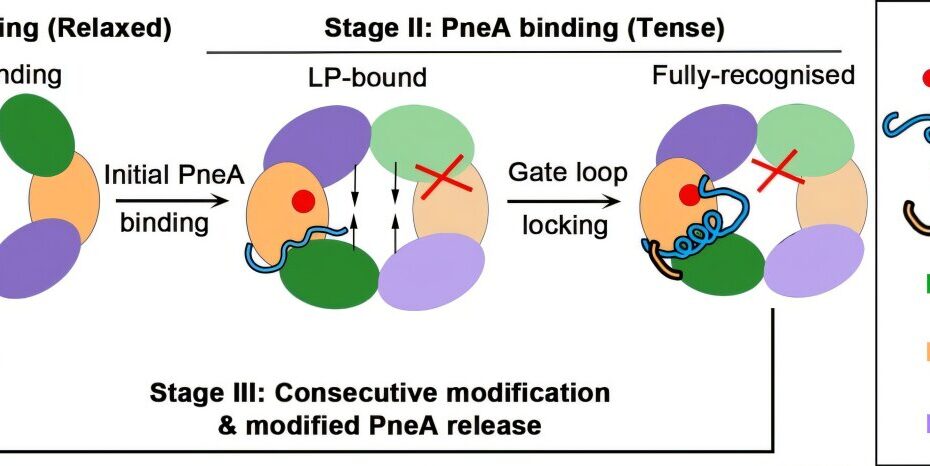
Using advanced techniques, including bioinformatics, cryo-electron microscopy (cryoEM), and functional assays, the team characterized a distinct subclass of lanthipeptide modification enzymes. These enzymes play a crucial role in converting a precursor peptide into its functional, biologically active form. They achieve this by sequentially installing a series of post-translational modifications that are necessary for the peptide to adopt its final, active conformation.
One of the major findings of this study was the discovery of the first homodimeric lanthipeptide modification enzyme, PneKC, derived from Streptococcus pneumoniae, a bacterium known for causing respiratory infections. The structural analysis of PneKC revealed a key region, or “dimerization hotspot,” where two parts of the enzyme come together to form a dimer. This dimeric structure is essential for the enzyme’s function, and when this region was mutated, the enzyme lost its ability to form the dimer, rendering it inactive.
The research team also found that the dimeric structure of PneKC operates through a process known as negative cooperativity. In this process, when a substrate binds to one part (protomer) of the dimer, it causes a conformational change that is transmitted to the unbound protomer. This change regulates the enzyme’s activity, ensuring that the peptide modification occurs in a controlled and sequential manner. This discovery of negative cooperativity provides new insights into how lanthipeptide modification enzymes regulate their activity and ensures that the peptide undergoes the correct series of modifications.
Additionally, the study identified a set of catalytic residues—specific amino acids within the enzyme’s active site—that play a pivotal role in facilitating the final step of peptide modification. This step, known as cyclization, is a critical process in the maturation of the peptide into its active lanthipeptide form. Prior to this study, the catalytic residues responsible for driving cyclization had not been identified, leaving a significant gap in the understanding of how these enzymes function.
The identification of these residues is a major breakthrough, as it provides the missing piece of the puzzle for understanding how lanthipeptide modification enzymes carry out the complex modifications required for peptide maturation. This discovery not only enhances the fundamental knowledge of enzyme function but also has practical implications for developing lanthipeptides for therapeutic use, particularly as antimicrobial agents.
Following the discovery of this new subclass of enzymes, Ms. Yifan Li, a Ph.D. candidate and the first author of the study, is continuing her research to explore the next stages of lanthipeptide modification. She is focused on understanding the later stages of peptide maturation and the pharmacodynamics of the newly identified lanthipeptides. This research is vital for unlocking the full therapeutic potential of lanthipeptides, particularly in the development of new antimicrobial drugs that can address the growing threat of antibiotic resistance.
Professor Luo Min emphasized the importance of these findings, noting, “Our current study significantly enhanced our understanding of the dynamic processes underlying peptide recognition and domain coordination within lanthipeptide modification enzymes. Moreover, considering the high potential of lanthipeptides in antimicrobial applications, these insights pave the way for the development of lanthipeptides for antimicrobial purposes.”
This discovery marks a significant advancement in the field of biochemistry and antimicrobial research. By providing a detailed understanding of how lanthipeptide modification enzymes function, the team has opened the door to the development of new, more effective antimicrobial drugs. As antibiotic resistance continues to rise, the ability to develop novel treatments based on naturally occurring compounds like lanthipeptides could become an essential tool in the fight against bacterial infections.
The research was published in the journal Nature Communications.
Source: National University of Singapore