A groundbreaking discovery by a team of researchers has resulted in a faster, more efficient method for producing nucleoside analogs, molecules vital for treating a range of diseases, from viral infections to cancer. This new process, described in a paper published in Nature Communications, promises to significantly accelerate drug discovery and development, making it easier to create new therapeutics with broad medical applications.
Nucleoside analogs are modified versions of nucleosides, the fundamental building blocks of DNA. These analogs are crucial in modern medicine due to their ability to interfere with cell replication processes, which is particularly useful in treating viral infections and cancer. According to Michael Meanwell, assistant professor in the Department of Chemistry and the corresponding author of the paper, nucleoside analogs have become integral in the development of treatments for a variety of diseases. “Nucleoside analogs are among the most important molecules to the advancement of modern medicine,” Meanwell explains. “They’re used as antivirals, as cancer therapeutics, in gene therapy. The first two drugs brought to market for treating COVID-19 were both nucleoside analogs.”
The research team focused on C4ʹ-modified nucleoside analogs, a subclass of these molecules. C4ʹ-modified nucleosides have garnered attention due to their potential as antiviral drugs, with some already undergoing clinical trials. However, their synthesis has been notoriously challenging. According to Meanwell, the difficulty lies in the chemistry used to create these compounds. “It’s mostly based on chemistry from 50, 60 years ago, and the advancements in modern organic chemistry have not yet been fully translated to this field of research,” he says. This has made it difficult for researchers to develop new nucleoside analogs efficiently and in a cost-effective manner.
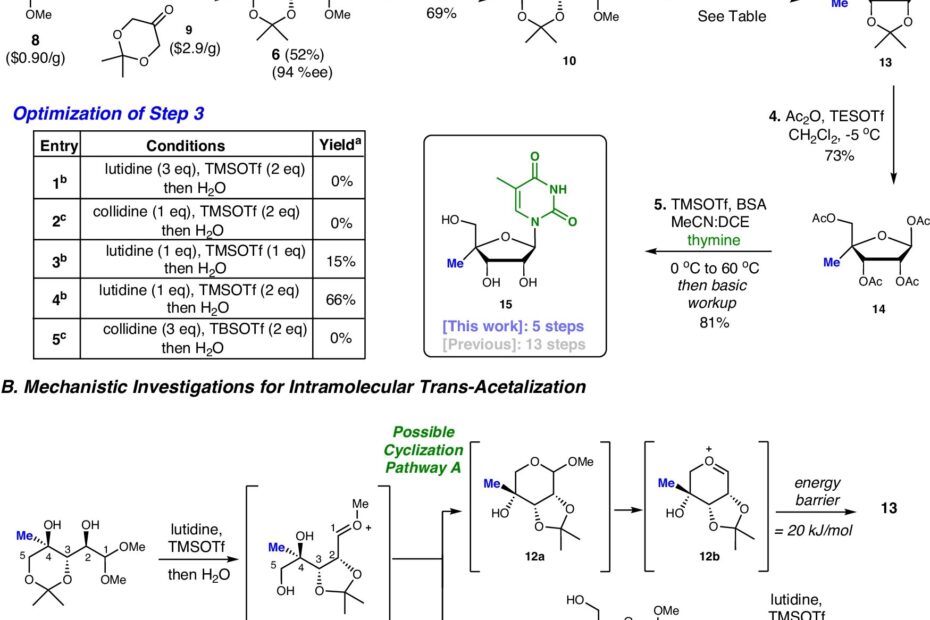
Traditional methods for producing C4ʹ-modified nucleoside analogs typically require anywhere from nine to 16 chemical steps. These approaches are not only time-consuming but also non-modular, meaning that to produce different analogs, entirely separate synthetic pathways need to be developed. This makes the process of creating a single molecule a lengthy task, often taking a month or more. In contrast, the new approach developed by Meanwell’s team requires only five steps and is modular in nature. This modularity allows researchers to create multiple nucleoside analogs simultaneously, greatly reducing the time and effort needed to synthesize these molecules.
“The breadth of molecules we can make with this one process is truly unprecedented,” says Meanwell. This advancement has significant implications for both basic research and pharmaceutical development. By streamlining the process, the barrier to drug discovery is lowered, especially for academic labs working with limited resources. Researchers can now experiment with a variety of potential therapeutics without being hindered by the complexity of synthesis.
The implications of this new method extend beyond simply reducing the time required for drug development. Nucleoside analogs are widely used in the treatment of various viral infections, including HIV/AIDS, hepatitis, Ebola, and respiratory syncytial virus (RSV). Their use is not limited to viral diseases; nucleoside analogs also play a role in cancer treatment. Cancer cells, much like viruses, rely on rapid cell division and replication, processes that can be inhibited by these modified nucleosides, thereby halting the growth of tumors.
“By inhibiting the same processes associated with cell growth and cell replication that cancer cells and viral diseases take advantage of, nucleoside analogs can treat those diseases,” says Meanwell. This ability to target fundamental biological processes makes nucleoside analogs powerful tools in the fight against a wide range of diseases. Over the years, they have been used to develop treatments for some of the most challenging and widespread infections.
The new approach not only offers a more efficient means of synthesizing C4ʹ-modified nucleoside analogs but also opens the door to creating a broader range of these therapeutics. “C4ʹ-modified nucleoside analogs are so important medicinally because of the wide range of disease pathologies they can intercept,” says Thirupathi Nuligonda, the lead author of the paper. “Now, we have a modular approach to synthesize this important class of nucleoside.” The flexibility of this new method allows for rapid testing and exploration of new therapeutic possibilities, making it a game-changer in both academic and clinical research.
The discovery also highlights a major shift in the way nucleoside analogs are produced. Traditionally, creating these compounds involved tedious, step-by-step chemical reactions, each step introducing the potential for error and inefficiency. The new modular process, however, reduces these complexities and allows for the creation of a variety of analogs using a more streamlined and predictable approach. This innovation has the potential to vastly improve the efficiency of drug development, particularly in the field of antiviral therapies and cancer treatments.
The impact of this breakthrough could be felt across a wide array of medical conditions. The versatility of nucleoside analogs means they could play a role in treating not only existing diseases but also emerging infections, like new strains of viruses. Their potential to interfere with the replication of various pathogens and abnormal cells makes them an invaluable tool in modern medicine. For example, the antiviral drugs that have been developed for the treatment of COVID-19 were both nucleoside analogs, underscoring the relevance and importance of this class of molecules in addressing global health challenges.
In addition to their antiviral and anticancer properties, nucleoside analogs have potential applications in gene therapy. Gene therapy involves altering the genetic material inside a person’s cells to treat or prevent disease, and nucleoside analogs can play a key role in this process. By modifying the structure of DNA and RNA, these analogs can be used to correct genetic defects or to inhibit the expression of harmful genes. Their use in gene therapy could pave the way for novel treatments for genetic disorders and other complex diseases that currently have limited therapeutic options.
The team’s discovery is an important step toward making the synthesis of nucleoside analogs more accessible and efficient. By simplifying the production process and making it modular, researchers are now able to explore a wider range of analogs and their potential therapeutic effects. This could accelerate the pace of drug discovery, ultimately leading to the development of more effective treatments for a variety of diseases.
As Meanwell notes, “It sounds like hyperbole, but they can treat such a broad range of diseases. They really stretch across so many different areas of health.” The ability to make nucleoside analogs more efficiently could ultimately transform the landscape of medicine, opening up new possibilities for treating infectious diseases, cancer, genetic disorders, and much more. By providing a faster and more scalable method of synthesis, this new approach could be the key to unlocking the next generation of life-saving drugs.
Source: University of Alberta