Scientists from Karlsruhe Institute of Technology (KIT) and the Indian Institute of Technology Guwahati (IITG) have pioneered a groundbreaking material with extraordinary water-repelling capabilities, opening up possibilities for advanced self-cleaning surfaces. Through an innovative process, they have transformed metal-organic frameworks (MOFs)—engineered materials known for their unique structural properties—by grafting hydrocarbon chains onto their surfaces. The result is a superhydrophobic material with water contact angles exceeding 160 degrees, making it ideal for applications requiring robust, water-resistant surfaces.
The research, recently published in the journal Materials Horizons, represents a significant leap in materials science. MOFs are networks made from metals and organic linkers that create a porous, sponge-like structure. These materials are prized for their vast surface area; a mere two grams of MOFs could cover an area as large as a football field. This exceptional property has made them invaluable for applications such as gas storage, carbon capture, and medical technologies. However, the research team’s novel method focuses on the exterior surfaces of these crystalline materials, exploiting their unique properties to achieve unprecedented water-repelling effects.
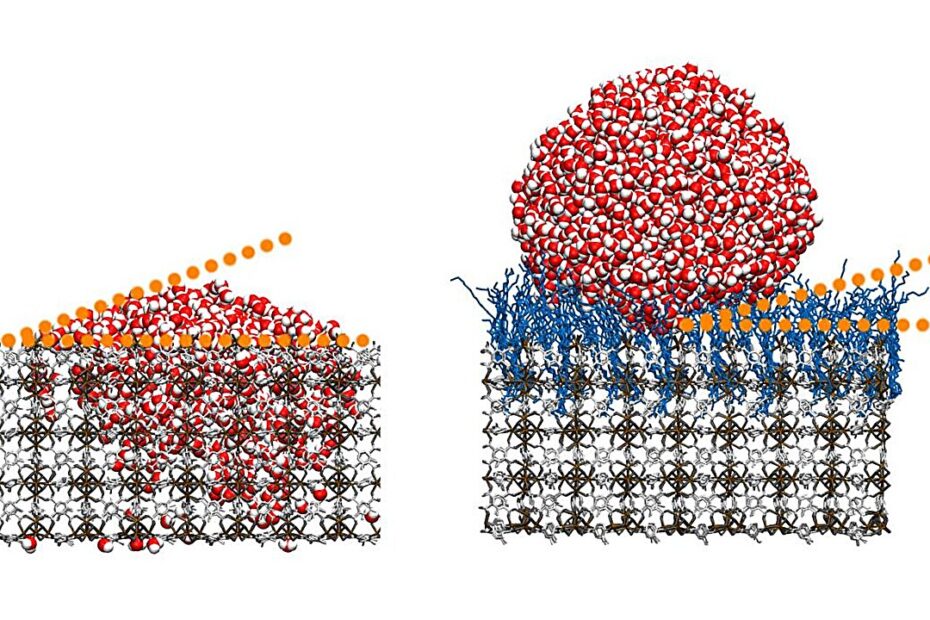
By grafting hydrocarbon chains onto thin films of MOFs, the scientists created a brush-like molecular arrangement, where the hydrocarbon chains adopt a disordered “high-entropy state.” This arrangement plays a critical role in the material’s superhydrophobicity. Unlike smooth surfaces that traditionally rely on coatings to repel water, these modified MOFs achieve superior water resistance intrinsically. Water droplets form minimal contact with the surface, resulting in a remarkable self-cleaning effect. This feature makes the material promising for applications in architecture, automotive industries, and other areas where durability and environmental resilience are essential.
Professor Christof Wöll from KIT’s Institute of Functional Interfaces emphasized the novelty of the approach: “With our method, we are able to achieve superhydrophobic surfaces with contact angles that are significantly higher than those of other smooth surfaces and coatings. Although the wetting properties of MOF powder particles have been explored before, the use of monolithic MOF thin films for this purpose is a groundbreaking concept.”
The team’s findings also shed light on the surprising role of molecular structure in achieving these hydrophobic properties. They discovered that substituting hydrogen atoms in hydrocarbon chains with fluorine—an approach typically used in materials like Teflon to enhance water resistance—actually reduced the material’s water contact angle. This result contradicted expectations, as perfluorinated materials usually enhance hydrophobicity. Further analysis using computer simulations revealed that perfluorinated molecules were unable to adopt the high-entropy state crucial for extreme water repellency, unlike hydrocarbon chains.
In another innovative step, the scientists manipulated the surface roughness of their SAM@SURMOF (self-assembled monolayers on surface-mounted MOFs) systems at the nanometer scale. This modification significantly reduced water adhesion, allowing droplets to roll off the surface even at extremely small inclination angles. The combination of surface roughness and the high-entropy molecular state amplified the material’s self-cleaning and hydrophobic properties, making it far superior to existing technologies.
Professor Uttam Manna of IITG highlighted the theoretical depth of the study: “Our work also includes a detailed theoretical analysis, which links the unexpected behavior shown in experiments to the high-entropy state of the molecules grafted to the MOF films. This study will change the design and production of next-generation materials with optimum hydrophobic properties.”
The implications of this research are far-reaching. Superhydrophobic materials have long been a focus of material science due to their potential applications in industries ranging from aerospace to medicine. For example, water-repellent surfaces could be used to prevent icing on aircraft wings, reduce fouling on marine vessels, or create highly efficient water-harvesting systems. In everyday contexts, they could enable self-cleaning windows, stain-resistant fabrics, and non-stick surfaces that maintain their properties even under harsh conditions.
Unlike traditional coatings that often degrade over time, the MOF-based approach offers a durable alternative that leverages the intrinsic properties of the material itself. This innovation aligns with the growing demand for sustainable and efficient materials in both industrial and consumer applications. The team’s emphasis on theoretical and experimental integration ensures that their findings not only demonstrate practical utility but also contribute to a deeper understanding of material behavior at the molecular level.
This research exemplifies the power of interdisciplinary collaboration, combining expertise in chemistry, materials science, and computational analysis. The partnership between KIT and IITG underscores the importance of international cooperation in addressing complex scientific challenges and developing transformative technologies.
Looking ahead, the researchers plan to explore further applications of their findings, including the integration of these materials into real-world systems. They are also investigating ways to enhance the scalability and cost-effectiveness of the production process, ensuring that this innovative technology can be widely adopted. With continued development, this breakthrough in MOF-based superhydrophobic materials could revolutionize industries, offering new solutions to longstanding challenges and inspiring future advancements in material science.