Microorganisms have relied on hydrogen as an energy source for billions of years, utilizing specialized enzymes called hydrogenases to catalyze its conversion. These hydrogenases, containing specific metals at their catalytic centers, allow microbes to efficiently process hydrogen without the use of precious metals or the release of greenhouse gases. Understanding the intricate mechanisms of hydrogen catalysis in these enzymes is a critical step toward harnessing their potential for sustainable hydrogen production and energy conversion. Recent advancements by a collaborative team of researchers have shed new light on these processes, marking a significant breakthrough in the field.
Hydrogen, often regarded as a cornerstone of a sustainable energy economy, holds promise as an alternative to fossil fuels. It serves as a clean energy carrier and a versatile catalyst in chemical processes. Despite its abundance on Earth, hydrogen is rarely found in its pure form. Instead, it exists predominantly in compounds such as water, natural gas, and crude oil. Extracting hydrogen from these compounds requires energy, posing economic and environmental challenges.
The most common industrial method for hydrogen production is steam methane reforming, which involves extracting hydrogen from natural gas. While efficient, this process produces significant amounts of carbon dioxide (CO₂), contributing to climate change. Alternatively, hydrogen can be produced through water electrolysis, a method that uses electricity to split water into hydrogen and oxygen. Although this method is cleaner, it often relies on electrodes made from precious metals like platinum, driving up costs and limiting widespread adoption.
Nature, however, offers an elegant alternative. Microorganisms, including certain bacteria and archaea, have evolved efficient methods to process hydrogen using three types of hydrogenases: [NiFe], [FeFe], and [Fe] hydrogenases. Unlike industrial processes, these enzymes function without expensive precious metals and do not emit CO₂. Among them, [Fe] hydrogenases are unique in their role within methanogenesis, a process that reduces CO₂ to methane (CH₄). This reaction is critical for many anaerobic archaea, and the enzyme’s catalytic mechanism has long intrigued scientists.
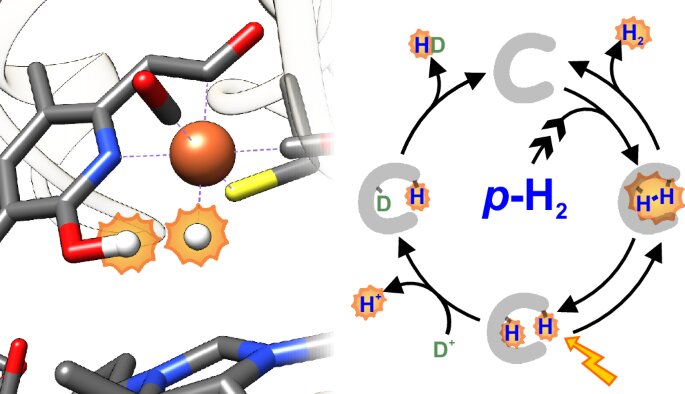
[Fe] hydrogenases are homodimeric enzymes, meaning they consist of two identical subunits. Each subunit contains a single redox-inactive iron (Fe) atom, which is bound to a guanylylpyridinol cofactor. While the catalytic intermediates of [NiFe] and [FeFe] hydrogenases have been extensively studied, those of [Fe] hydrogenases remained elusive due to their fleeting nature and the lack of suitable observation techniques.
This longstanding gap in understanding has now been bridged by a collaborative research effort. The team, comprising experts from three Max Planck Institutes, the Center for Biostructural Imaging of Neurodegeneration (BIN) at the University Medical Center Göttingen (UMG), Kiel University, and FAccTs GmbH, employed advanced nuclear magnetic resonance (NMR) spectroscopy techniques to investigate the catalytic cycle of [Fe] hydrogenases. Their findings offer unprecedented insights into the enzyme’s mechanism.
A key aspect of this breakthrough was the use of a chemical property of hydrogen. Hydrogen exists in two spin isomers, parahydrogen and orthohydrogen, which differ in the alignment of their nuclear spins. By leveraging parahydrogen-induced polarization (PHIP), the researchers achieved signal amplification in NMR spectroscopy, enabling them to detect reaction intermediates that were previously invisible. This approach allowed the team to visualize how [Fe] hydrogenases bind and process hydrogen during catalysis.
The study revealed that during the reaction, a hydride—a negatively charged hydrogen ion—is formed at the iron center of the enzyme. This discovery provides a detailed picture of the intermediate steps in the catalytic cycle. Moreover, the high sensitivity of the PHIP technique allowed the researchers to study the kinetics of hydrogen binding, opening new avenues for exploring hydrogen metabolism in living cells.
The implications of these findings are far-reaching. By unraveling the catalytic mechanism of [Fe] hydrogenases, scientists can pave the way for designing more efficient biocatalysts for hydrogen production and conversion. Unlike traditional methods that rely on costly materials, these bio-inspired catalysts could enable hydrogen production on a larger scale and at a lower cost, accelerating the transition to a sustainable energy economy.
The potential applications extend beyond hydrogen production. Understanding hydrogenase function could lead to advances in synthetic biology, where enzymes are engineered for specific industrial processes. Additionally, the ability to study hydrogen metabolism in vivo using PHIP could revolutionize research in microbial ecology, biomedicine, and renewable energy systems.
This breakthrough also highlights the power of interdisciplinary collaboration. By combining expertise in biochemistry, structural biology, and magnetic resonance spectroscopy, the team achieved a level of precision and insight that was previously unattainable. Their work underscores the importance of integrating cutting-edge techniques to address complex scientific challenges.
In the context of the global energy crisis and the urgent need to mitigate climate change, such advancements are particularly timely. Hydrogen has the potential to serve as a clean and efficient energy carrier, but its widespread adoption hinges on the development of cost-effective and environmentally friendly production methods. Nature’s hydrogenases, refined through millions of years of evolution, offer a blueprint for achieving this goal.
The results of this research not only deepen our understanding of microbial hydrogen metabolism but also provide a foundation for innovative technologies that could reshape the energy landscape. As scientists continue to explore the capabilities of hydrogenases and other biological systems, the line between biology and technology becomes increasingly blurred, opening new possibilities for sustainable development and energy solutions.
The study is published in Nature Catalysis.
Source: Max Planck Society